Radiopharmacy
"The incorporation of the new PET-CT, together with the use of radiopharmaceuticals, allows us to detect lesions that until now were practically impossible to identify".
DR. IVÁN PEÑUELAS DIRECTOR. RADIOPHARMACY UNIT

The Clinic's Radiopharmacy Unit is the only Spanish hospital with the capacity to synthesize and apply 20 different radiopharmaceuticals.
The benefit for our patients of this wide variety of radiopharmaceuticals is that it allows a more precise diagnosis to determine the most appropriate treatment for complex pathologies such as prostate, brain and liver tumors, degenerative diseases such as Parkinson's or Alzheimer's and dementias in general.
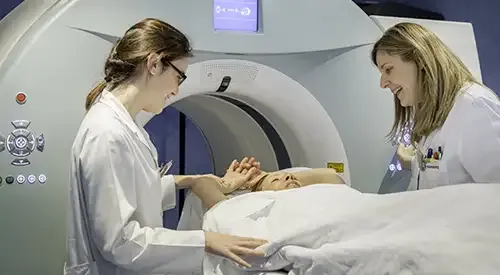
The radiopharmaceutical is used as a contrast compound that is injected into the patient to observe the interior of the body in vivo, in a non-invasive way. The objective is to obtain the molecular image of the organism itself or of the specific disease to be studied.
Our facilities for the elaboration of radiopharmaceuticals comply with GMP (Good Manufacturing Practices) conditions or internationally established standards of correct elaboration.
Therefore, we apply the maximum safety conditions in the production of the medicines, both in radiological protection and in the personalized dosage for each patient.

PET Service and Radiopharmacy Unit join forces to obtain maximum diagnostic and therapeutic accuracy
The Clinic is one of the European hospitals that has a greater number of specific radiopharmaceuticals for certain pathologies for clinical use in the center itself and about a fortnight in research and clinical trials
IN NAVARRE
The PET (Positron Emission Tomography) technique allows a very advanced and precise early image diagnosis in different pathologies, especially of tumors.
The singularity is that the metabolic changes that are detected by this technique, thanks to the activity of the radiopharmaceutical, are previous to the structural ones so it is possible to detect the alterations at a molecular level, before their anatomical perception.
Hence the possibility of a very early diagnosis of the disease that will result in a better prognosis. In addition, this technique also provides early information on the patient's response to treatment, by observing the modifications.
Technified radiopharmaceuticals
- 99mTc- Phytacis®
Technetium Phytate
Use: liver scan - 99mTc- Nanocis®
Rhenium Colloidal Sulfide
Utilization: lymphographies and gastroesophageal scintigraphy - 99mTc- MAG-3®
Benzoyl mercapto acetyl triglycine
Utilization: evaluation of nephrological and urological disorders - 99mTc- Bridatec®
Mebrofenin
Utilization: hepatobiliary imaging and hepatobiliary function studies - 99mTc-DMSA
Technetium Succuminum
Use: static renal imaging - 99mTc-PYP
Sodium pyrophosphate anhydrous
Utilization: red blood cell marking, blood volume determination and spleen scan - 99mTc-HDP
Oxydrate
Use: bone scan - 99mTc-MAA (Lyomaa®)
Albumin macro-aggregates
Utilization: lung perfusion scan and venography scan - 99mTc-Cardiolite®
Sestamibi
Use: diagnosis of decreased coronary perfusion, myocardial infarction, diagnosis of breast cancer malignancy and in hyperparathyroidism - 99mTc- Myoview®
Tetrofosmin
Utilization: cardiac and breast scan - 99mTc- Ceretec®
HMPAO (examethylene p-amino oxime, exametazim)
Use: brain scan and leukocyte marking with 99mTc - 99mTc-DTPA
Technetium Pentetate
Utilization: renal scan, cerebral angiogammagraphy, cerebral scan, pulmonary ventilation scan and gastroesophageal reflux and gastric emptying studies - 99mTc- Ceretec® stabilized
HMPAO (examethylene p-amino oxime, exametazim)
Utilization: brain scaN - 99mTc- Vasculocis®
HSA (human serum albumin)
Bone marrow imaging, areas of abdominal swelling, and lymphatic system integrity study - 99mTc- Nanocoll®
Colloidal human albumin
Utilization: vascular phase imaging, angiocardiography and ventriculography
Non-technical radiopharmaceuticals
- 90Y- ZEVALIN®
Ibritumomab tuxetan
Utilization: treatment of adult patients with non-Hodgkin's lymphoma - 111In- Octeoscran®
PenteotridUtilization: diagnosis of carcinoid and gastro-entero-hepatic neuroendocrine tumors
Do you need to request a consultation with one of our specialists?
DIAGNOSIS AND PERSONALIZED TREATMENT
Pioneers in technology
The Clinic has become the pioneering Spanish center in the field of Radiopharmacy, especially in its use with the PET technique.

Radiopharmaceuticals
Approximately 95% of radiopharmaceuticals are used for diagnostic purposes and therefore their application does not have a therapeutic purpose like regular drugs. Each radiopharmaceutical has a recommended dose range for each of the authorised clinical indications.

Quality and Safety
Radiopharmaceuticals are the safest medicines available because, in addition to the rigorous controls to which they are subjected, the doses administered to patients are so low that there is no possibility of adverse effects.

Unique facilities
GMP installations comply with a unidirectional flow of materials and products. The laboratory guarantees radiological protection, both for the personnel working in the radioactive facility and for the rest of the Clinic's professionals and the public in general.

Why choose Navarra?
- Precise diagnosis thanks to the combination of PET and radiopharmaceutical.
- National references professionals in this type of technique.
- We research together with the rest of the Clinic's departments in order to offer therapeutic alternatives to our patients.
Our team of professionals
Good Manufacturing Practices, internationally established standards of good manufacturing
Highest quality
Nuclear Medicine GMP Laboratory
The implementation of this PET GMP laboratory, in 2009, obtained the approval of the Department of Innovation, Business and Employment of the Government of Navarra, with the favourable report of the Nuclear Safety Council (CSN).
The laboratory is subject to inspections by the Government of Navarra's Department of Health and the CSN.
We help our patients to overcome their stories
Their testimonies encourage us to continue improving our services







